
The kinetic parameters optimized from deactivation were described as Arrhenius equation. The experiments were done at 565 to 600 to 635 ☌ and atmospheric pressure with the steam ratios of 1.5, 2.2 and without steam. Moreover, the main reaction kinetic model, a novel deactivation one was presented included various coke formation, sintering, dealumination and steam effect were studied in detail. However, steam dilution led to higher dealumination rate and sintering which results in activity reduction. In this study, steam was injected accompanied with feed to decrease the coke accumulated on catalyst surface and reduce the deactivation rate. Coke deposition has a significant contribution in catalyst deactivation. One of the major issues associated with the catalytic cracking reaction is the activity decrement during time on stream.

For the catalytic cracking of LPG, the mathematical modeling based on a complex reaction methodology was proposed. The purpose of this work was to propose a kinetic model for liquefied petroleum gas (LPG) catalytic cracking over modified ZSM-5. The study demonstrated that ethylene/propylene ratio (E/P) is impacted by zeolite structure which is affected by the steam addition during the reaction. In the presence of steam, ZSM-5 pore-shape modification favored the monomolecular cracking pathway leading to achieving higher light olefin yield than that observed over BEA zeolite. During catalytic cracking in the absence of steam, hydrogen transfer reaction was less favored over BEA zeolite, which result in higher light olefins selectivity and dry gas formation than with ZSM-5 zeolite. However, the zeolites structural modification has shown significant effect on the pore shape selectivity which result in different light olefins yields. In addition, the steam addition has significantly affected the zeolites structure, even though their cracking activity was not affected. Although both synthesized submicron catalysts (400–500 nm) have similar Brønsted/Lewis acid ratios, the BEA zeolite exhibited higher surface acidity than ZSM-5 zeolite. The reaction utilized the different 3D sinusoidal microporous channels in submicron ZSM-5 and BEA zeolite crystals to convert dodecane to light olefins.
In this study, steam catalytic cracking of dodecane (a model representative of naphtha) was investigated both with and without steam. On the other hand, whilst steam does not modify ethene and propene selectivity, significantly decreases H2 and CH4 formation, as well as formation of potential coke precursors.
It appears that whilst the presence of steam is vital when processing heavy feeds to achieve a better feed dispersion and a more effective catalytic cracking in conventional fluid catalytic cracking (FCC) units, in the case of steam catalytic cracking of naphtha (n-heptane) the presence of steam has a negative effect on the final performance of the catalyst. The apparent activation energy is lower in the presence of steam. A kinetic decay model that takes into account the two phenomena has been developed. It has been found that under those conditions the presence of steam produces an irreversible dealumination of the zeolite as well as a reversible deactivation due to the interaction of water with active sites with a negative effect on protolytic cracking. We present here the results obtained for high temperature steam catalytic cracking (SCC) of a representative naphtha product (n-heptane) with ZSM-5.
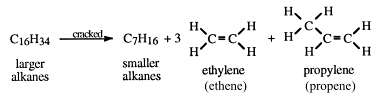
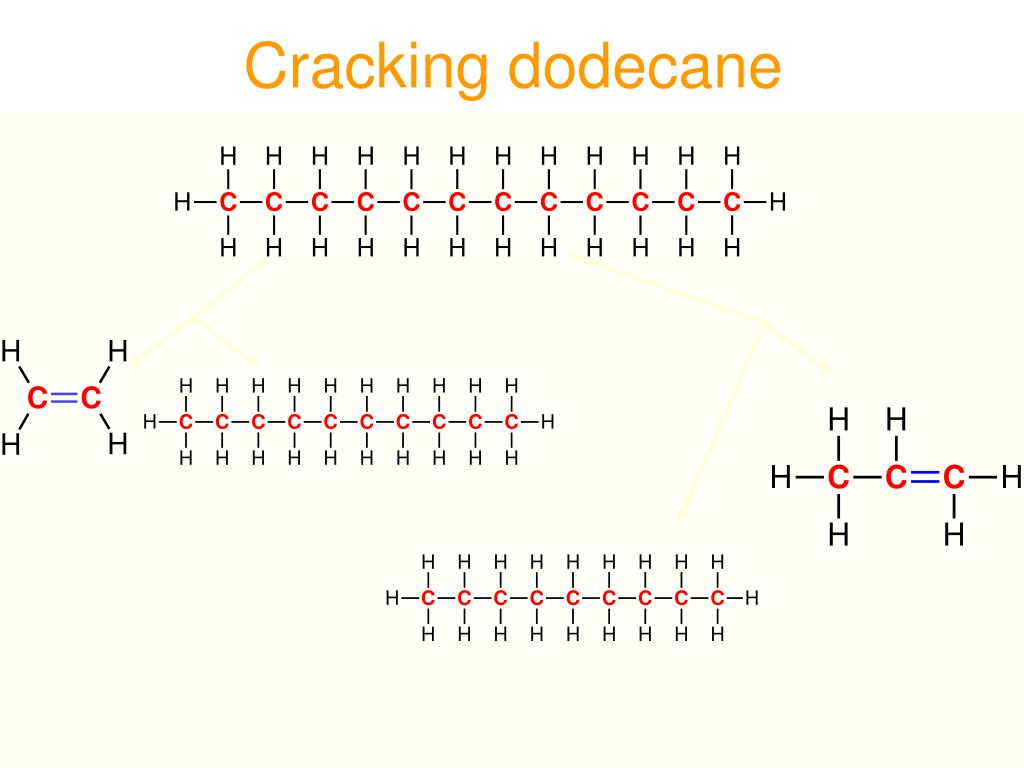
Cracking of dodecane to make ethene crack#
One option to produce more ethene and propene can be to crack naphtha type fractions in dedicated smaller FCC units.


 0 kommentar(er)
0 kommentar(er)
